Which One of the Following Is a Strong Acid
Weak acid partially ionized. Each of the following pairs contains one strong acid and one weak acid except.

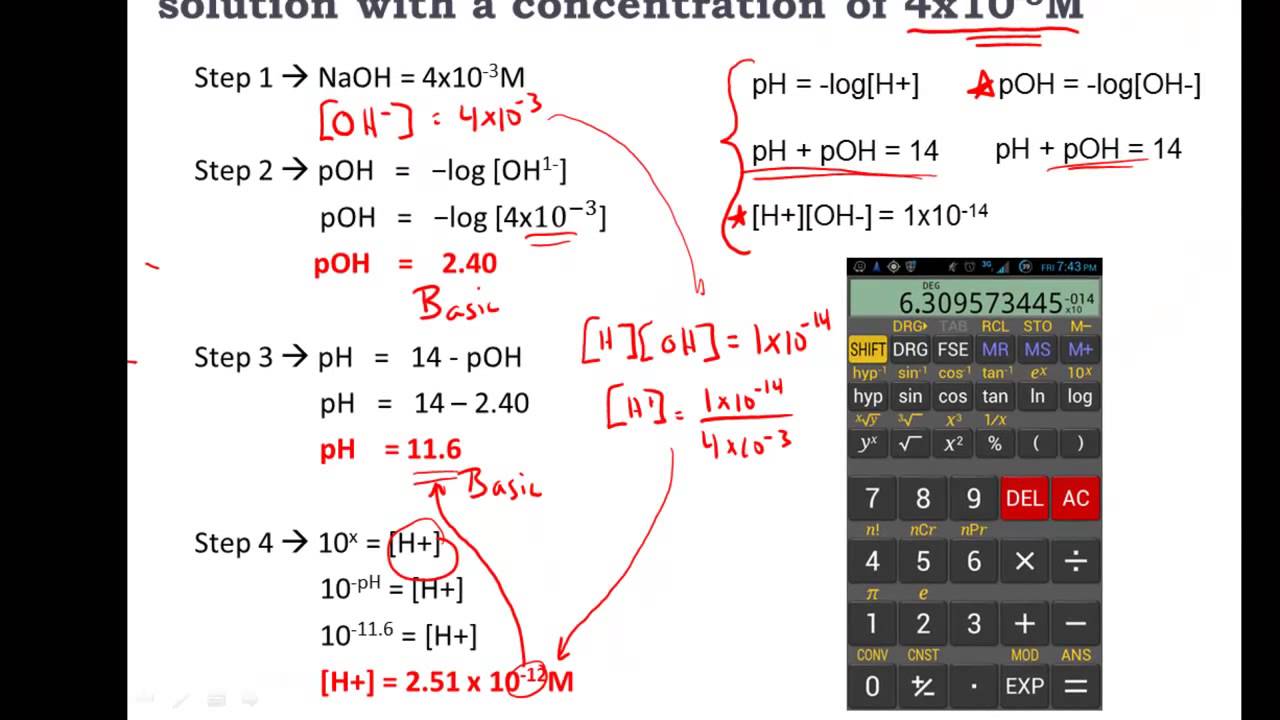
Pin On Chemistry Analytical Chem
Here some of you may think that the higher the value of pH shows the highest strength of acidity but this is not true.

. Nitric acid also known as aqua fortis is a highly corrosive mineral acid. Review the branily terms and services guides well u know what i never did so go have a nice ice cream sunday. A HNO3 and H2CO3 b HI and HBr c H2SO4 and H2S d HCl and HC2H3O2 e HBr and H3PO4.
Which one of the following statements about sulfuric acid is correct. Sulfuric acid H2SO4 is a strong acid. A weak acid is the opposite of a strong acid A strong base is the opposite of a strong acid A neutral solution is the.
Most salts are neutral substances in some cases salts may show acidic or basic or amphotoricboth acidic or basic characteristics. Strong electrolyte aq cation aq anion aq. C C l 3 C O O C H C l 2 C O O C H 2 C l C O O C H 3 C O O So strongest acid is C C l 3 C O O.
HCl hydrochloric acid H 2 SO 4 NaOH and KOH are all strong electrolytes. See the answer See the answer See the answer done loading. Which one of the following is a strong acid.
Strong soluble base soluble metal hydroxide. We review their content and use your feedback to keep the quality high. Answer 1 of 2.
It depends which part of the stong acid concept you are concerned with. Strength of Conjugate base order. HCl is the acid.
Weak base accepts H from an acid. Strong acid means weak conjugate base. Which one of the following is a strong acida sulfuric acid H2SO4b phosphoric acid H3PO4c hydrofluoric acid HFd acetic acid HC2H3O2 This problem has been solved.
It is fully dissociated in diluted solution except in extremely acidic solutions. 2 Get Other questions on the subject. If that is the case you are comparing trichloroethanoic acid to tribromoethanoic acid and the stronger one is the first one.
Nitric acid dissociates completely in its aqueous solution. Which one of the following will give a solution with a pH 7 but is not an Arrhenius base in the strict sense. Which one of the following is a strong acid.
Thus it is a strong acid. Salt cation of an acid anion of a base. Complete the following reaction and identify the Brønsted acidNaOHaq HClaq A.
A lower value of pH shows the highest strength of an acid and as the value of pH increases acidity decreases and above 7 basicity increases. The substance NH3 is considered. Strong electrolyte strong acid strong base or soluble salt.
Sulfuric acid has little effect on metals. Which one of the following is a strong acid. Which term is not correctly matched with its description.
NaOH is the acid. It is a strong oxidizing agent and a strong acid at ambient temperature. Because three Cl atoms on the C next to the -COOH group exert a.
Which one of the following is a strong acid. The general form of the strong electrolyte equation is. H2CO3 H2SO3 H2SO4 H3PO4 CH3COOH H2SO4.
HCl is the acid. See what the community says and unlock a badge. From the above discussion it is clear that Acetic acid is not a strong acid.
Is salt and acid or base. Sulfuric acid is a known muriatic acid. Chemistry 21062019 1600 ntrann22.
Vinayak can also be set to get a option so we are. It behaves as typical acid in its reaction with most metals. Which one of the following is not a strong acid.
Sulfuric acid is dangerous to living organisms. Plz mark as Brainliest Dear. Experts are tested by Chegg as specialists in their subject area.
Which one of the following is strong acid. HNO3 is also called nitric acid is a strong acid which dissociates into H and NO3- H2SO4 is one of the strongest acid available in a lab. NaOH is the acid.
NaCl is the acid. The substance CH3CH22NH is considered. I suppose you meant Br₃CCOOH rather than BrCCOOH.
Sulfuric acid is a strong oxidizing agent. It has two a. Hello in the question that which one of the following and strong acid and weak acid and carbonic acid on what factors does acidity emotional to dissociation power dissociation amount of dissociation defence this facility is directly proportional to the stability of anion.
So the correct answer is Option B.

Pin By Pawan Jangid On Chemistry Chemistry Notes Science Chemistry Chemistry

Pin By Carin Barber On Chemistry Notes In 2021 Chemistry Notes Non Electrolyte Chemistry

Strong Acids And Bases Mcat Chemistry Cheat Sheet Study Guide Studypk Chemistry Lessons Teaching Chemistry Chemistry Classroom

Acids Bases And The Ph Scale Quiz Teaching Chemistry Quiz Base

Mechanism Challenge 1 Organic Chemistry Tutor Chemistry Chemistry Lessons

Pin On Change Your Water Change Your Life With Kangen Water

Dust Filtration Filter Material How To Make Oil Fluidized Bed Anhui

The Ordinary Skincare A Guide To The Best Ingredients Prettygalslove The Ordinary Skincare Beauty Skin Care Routine Skin Care Routine Steps

Partial Pressure Easy Science Study Skills Chemical Changes Easy Science

Formation Of Alkoxide Ions Organic Chemistry Books Chemistry Notes Chemistry







Comments
Post a Comment